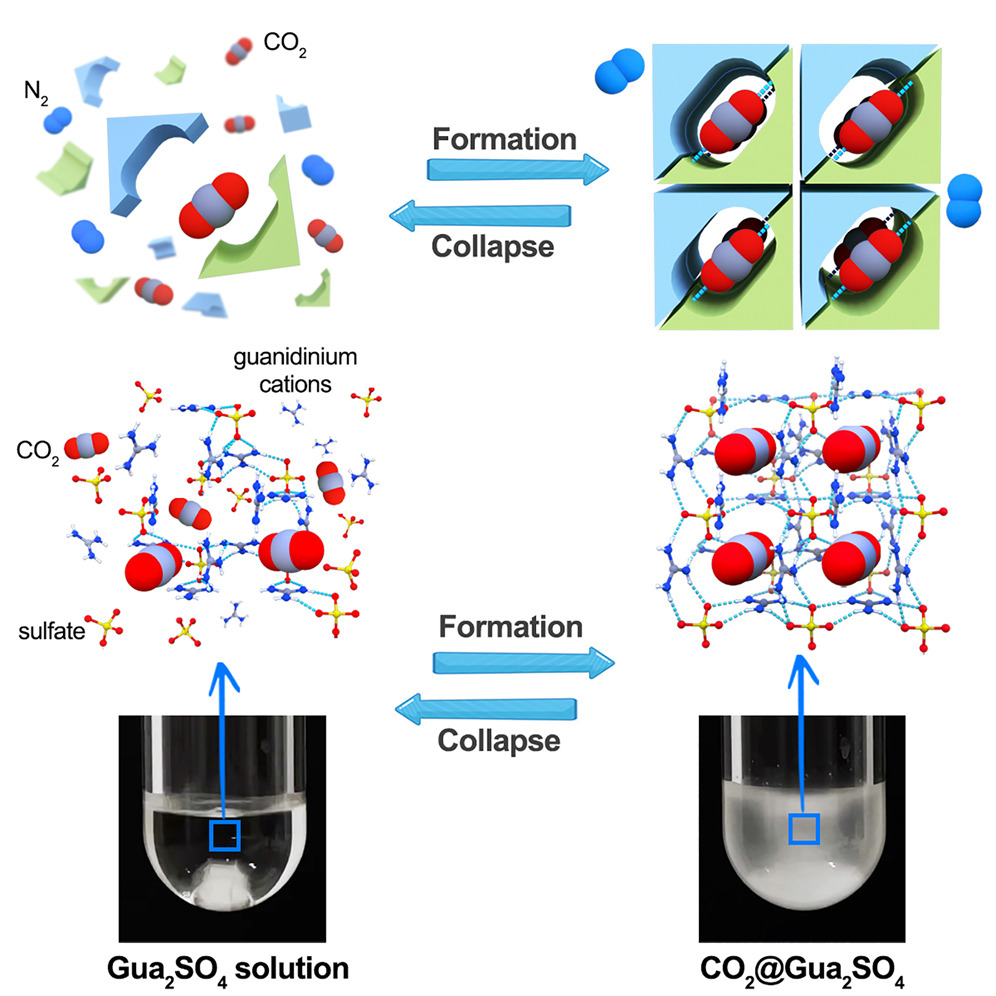
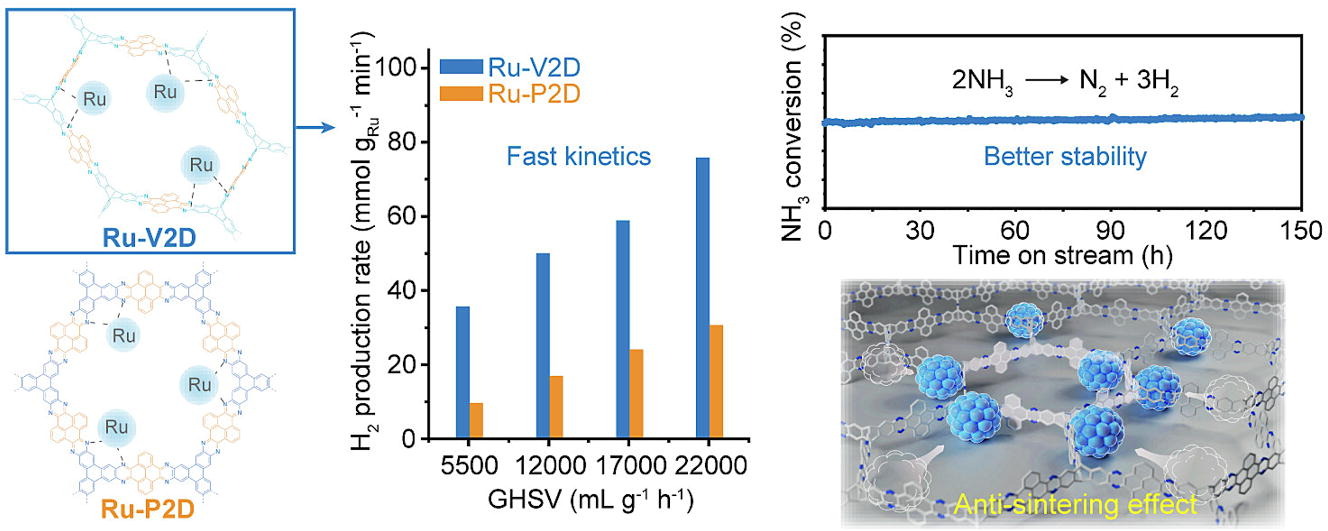
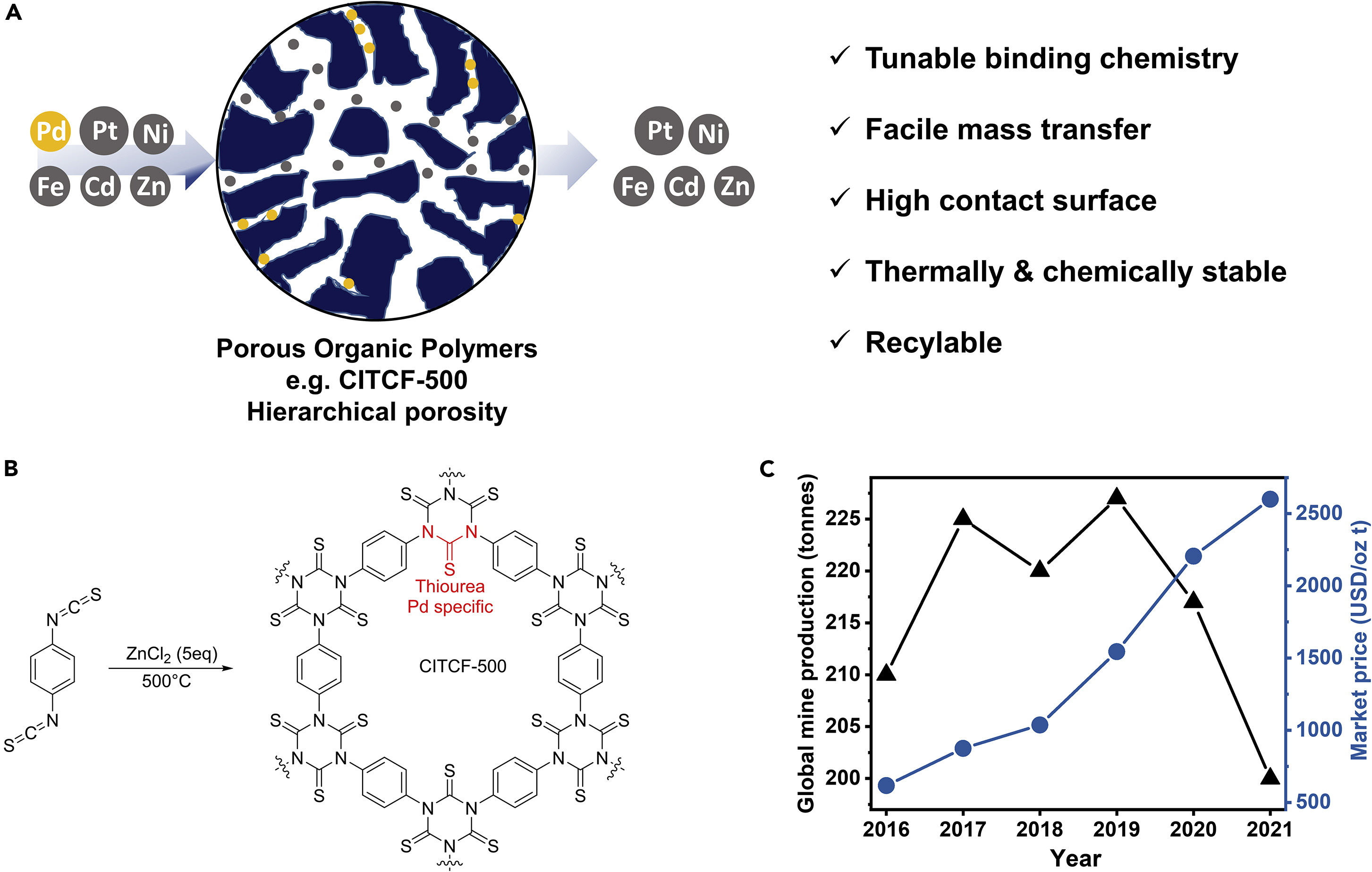
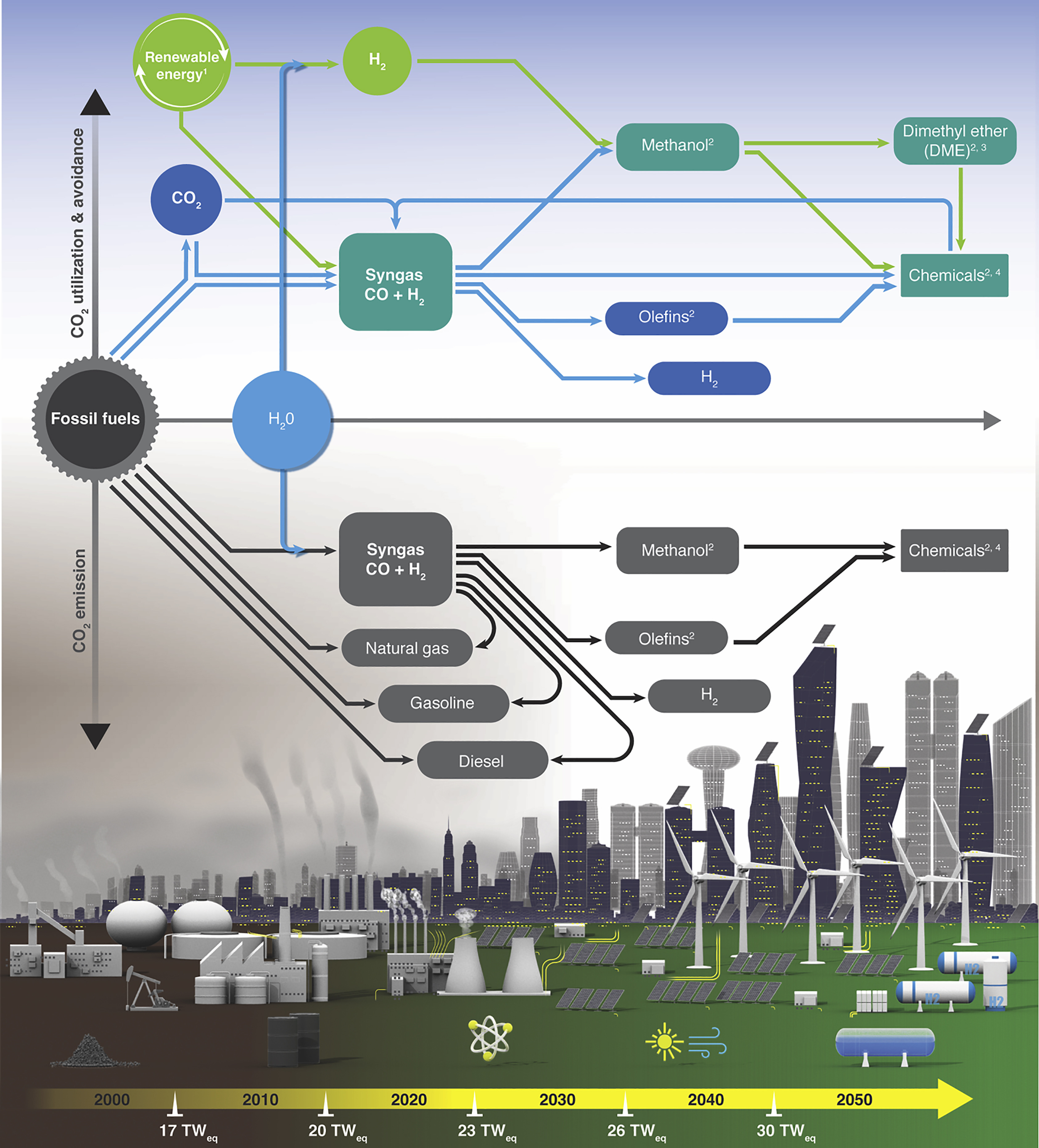
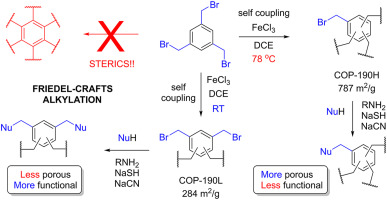
Porous organic polymers with labile leaving groups offer direct access to reactive functional groups, otherwise not permissible during network formation. In a one-step, open air, self-coupling reaction of tris bromomethyl benzene, we report highly porous, bromine rich C–C bonded porous polymers. Due to the steric nature of the monomer, restrictive crosslinking allowed pendent bromine groups to remain unreacted and provided rapid exchange into amines, nitriles, and thiols. This simple but powerful strategy yielded two isostructural but varying porosity and pendent group density polymers, allowing a comparative gas uptake study. Despite having lower surface area, the porous polymer formed at low temperature showed higher amination due to higher density of bromine groups. The polymers with more pendant groups resulted better CO2 uptake performances than higher porosity polymers with less pendant groups. Although post-modification decreased surface area of materials, amine functionalization greatly improved the CO₂ uptake capacity. The ethylenediamine appended version exhibited 4.7 times increase in CO₂ uptake capacity with highest CO₂/N₂ selectivity of 729 (298 K), and with an isosteric heat of 97 kJ mol−1 at zero coverage.