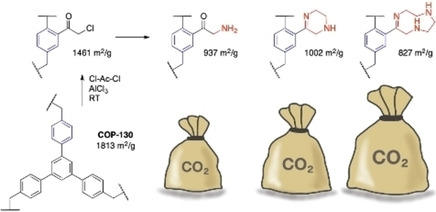
Effective removal of excess greenhouse gas CO2 necessitates new adsorbents that can overcome the shortcomings of the current capture methods. To achieve that, porous materials are often modified post-synthetically with reactive amine functionalities but suffer from significant surface area losses. Herein, we report a successful amine post-functionalization of a highly porous covalent organic polymer, COP-130, without losing much porosity. By varying the amine substituents, we recorded a remarkable increase in CO2 uptake and selectivity. Ketone functionality, a rarely accessible functional group for porous polymers, was inserted prior to amination and led to covalent tethering of amines. Interestingly, aminated polymers demonstrated relatively low heats of adsorption, which is useful for the rapid recyclability of materials, due to the formation of suspected intramolecular hydrogen bonding.