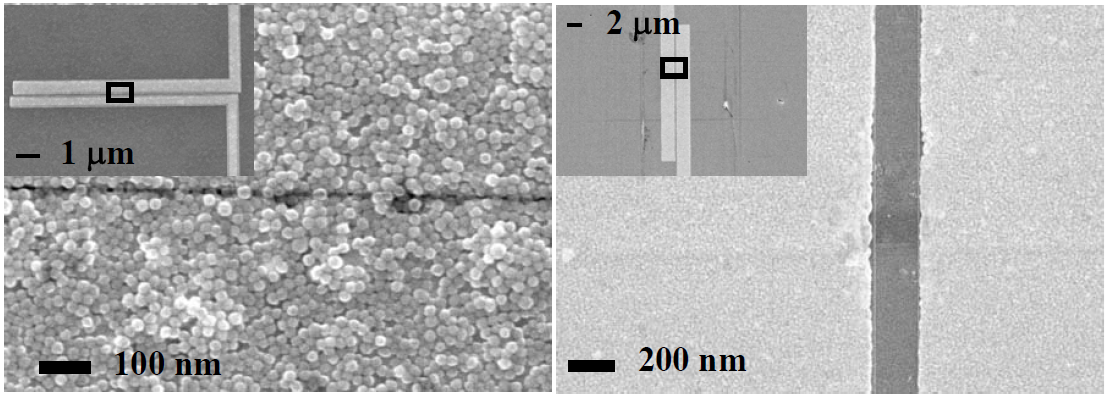
Magnetite (Fe3O4), an archetypal transition-metal oxide, has been used for thousands of years, from lodestones in primitive compasses1 to a candidate material for magnetoelectronic devices2. In 1939, Verwey3 found that bulk magnetite undergoes a transition at TV≈120 K from a high-temperature ‘bad metal’ conducting phase to a low-temperature insulating phase. He suggested4 that high-temperature conduction is through the fluctuating and correlated valences of the octahedral iron atoms, and that the transition is the onset of charge ordering on cooling. The Verwey transition mechanism and the question of charge ordering remain highly controversial5,6,7,8,9,10,11. Here, we show that magnetite nanocrystals and single-crystal thin films exhibit an electrically driven phase transition below the Verwey temperature. The signature of this transition is the onset of sharp conductance switching in high electric fields, hysteretic in voltage. We demonstrate that this transition is not due to local heating, but instead is due to the breakdown of the correlated insulating state when driven out of equilibrium by electrical bias. We anticipate that further studies of this newly observed transition and its low-temperature conducting phase will shed light on how charge ordering and vibrational degrees of freedom determine the ground state of this important compound.